#1
Meera Srikrishna
CT-based imaging markers for idiopathic normal pressure hydrocephalus obtained using deep learning: association with MRI-based radiological markers and diagnosis
Background: Brain computed tomography (CT) is a widely accessible and affordable modality routinely used to assess neuroimaging signatures of idiopathic normal pressure hydrocephalus (iNPH), particularly ventricular enlargement. Previously, we developed deep learning models to segment various brain tissue classes in brain CTs. This study aims to develop novel tools capable of automatically extracting volumetric measurements and ratios from CT brain scans. Further, we aim to assess the association of CT-based volumetric measures (CTVMs) with radiological markers of iNPH and diagnosis.
Methods: We included 777 (52% female, 70.44 ± 2.6 years) CT scans which had paired T1-weighted magnetic resonance image scans from the Gothenburg H70 Birth Cohort studies collected on the same day. An experienced rater evaluated various iNPH-related radiological markers in all T1-weighted images. Thirty-nine participants fulfilled the criteria of radiological probable iNPH. Our trained U-Net-based deep learning models were used to predict brain volume (BV; obtained by the summation of grey matter (GM) and white matter (WM) tissue classes), intracranial volume (ICV), cerebrospinal fluid (CSF), and ventricular CSF (VCSF) segmentations from input CT scans. CT-based volumetric measures (CTVMs) such as VCSF/ICV, BV/ICV, and VCSF/CSF were derived. The relationship between CTVMs and standard radiological markers such as Evan’s index, Z-Evan’s index, and callosal angle was assessed using Spearman’s rank correlation tests.
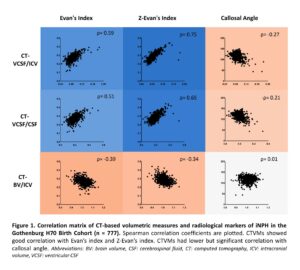
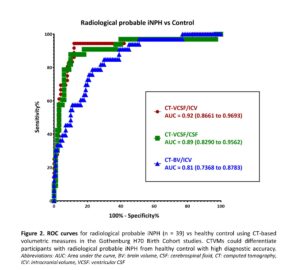
Results: CT-based volumetric measures could differentiate participants with radiological probable iNPH from healthy control with high diagnostic accuracy (Area under the curve: 0.92 for CT-VCSF/ICV). CTVMs showed good correlation with Evan’s index (ρ=0.59, p<0.001) and Z-Evan’s index (ρ=0.75, p<0.001). CTVMs had a lower but significant correlation with callosal angle (ρ=-0.27, p<0.001). CT-VCSF/ICV showed a higher correlation with radiological markers of iNPH in comparison to CT-BV/ICV and CT-VCSF/CSF. Higher levels of Evan’s index and Z-index are associated with higher levels of CT-VCSF/ICV and CT-VCSF/CSF and lower levels of CT-BV/ICV.
Conclusion: CT-based volumetric measures correlate with relevant radiological markers of iNPH. CTVMs could distinguish radiological probable iNPH and controls. This supports the potential application of CTVMs in aiding diagnostics and monitoring the progression of iNPH. Automated CT-based angular measurements and the association of CTVMs with other clinical iNPH markers will be further explored in future.
Authors: Meera Srikrishna1,2,3, Clara Constantinescu4,5, Doerthe Ziegelitz6, Anna Zettergren7, Silke Kern7,8, Lars-Olof Wahlund9, Eric Westman9, Ingmar Skoog7, Mats Tullberg4, Michael Schöll1,2,3,10,11
Author Affiliations:
- Centile Bioscience, USA & UK;
- Wallenberg Centre for Molecular and Translational Medicine, University of Gothenburg, Sweden;
- Department of Psychiatry and Neurochemistry, Institute of Physiology and Neuroscience, University of Gothenburg, Sweden;
- Department of Clinical Neuroscience, Institute of Neuroscience and Physiology, The Sahlgrenska Academy, University of Gothenburg, Sahlgrenska University Hospital, Sweden;
- Department of Neurology, Sahlgrenska University Hospital, Sweden;
- Department of Radiology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Sahlgrenska University Hospital;
- Neuropsychiatric Epidemiology, Institute of Neuroscience and Physiology, Sahlgrenska Academy, Centre for Ageing and Health (AgeCap), University of Gothenburg, Sweden;
- Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Mölndal, Sweden;
- Division of Clinical Geriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Sweden;
- 1Dementia Research Centre, Institute of Neurology, University College London, UK;
- Department of Clinical Physiology, Sahlgrenska University Hospital, Gothenburg, Sweden