#5
Jacob Vogel
An update on Alzheimer’s disease tau subtypes: clinical and biological insights
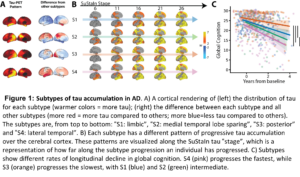
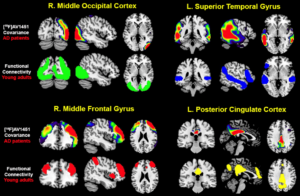
Background: The aggregation of tau pathology in Alzheimer’s disease (AD) is strongly associated with cognitive decline and local neurodegeneration. Tau pathology accumulates in the cerebral cortex in complex spatiotemporal patterns that differs across individuals, leading to diverse clinical phenotypes. Using positron emission tomography (PET), we recently characterized four subtypes of tau accumulation within the AD population, each with distinct clinicopathological profiles (Fig 1). Validation and further characterization of these subtypes is critical to better understand their clinical and potential therapeutic relevance.
Methods: We applied the previously established Subtype and Stage Inference (SuStaIn) model to 1252 BioFINDER-2 (BF2) participants with available [18F]RO948 tau-PET. Linear models assessed subtype differences in CSF-biomarkers (corrected for Aβ40 and log-transformed). Linear mixed models were used to determine regional subtype-differences in atrophy (gray-matter volume) and cognition (MMSE and ADAS-cog delayed recall) over time. Models included age, sex, and baseline diagnosis, TIV (atrophy) and level of education (cognition) as covariates and were FDR-corrected.
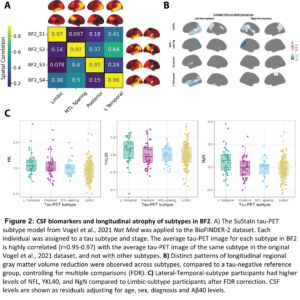
Results: The four previously identified tau-PET subtypes were replicated in BF2 (Fig. 2A). Participants classified to the Limbic-subtype were older (F=33.3, p<0.01), while Posterior-subtype participants were less often APOE-ε4 carriers (χ2=119.83, p<0.001). Distinct patterns of longitudinal regional gray matter volume reduction were observed across subtypes (Fig. 2B). Posterior and Lateral-Temporal-subtype participants had higher levels of NFL, YKL40, and NgN compared to Limbic-subtype participants (pFDR<0.05, Fig. 2C). Limbic-subtype and Lateral-Temporal-subtype participants performed worse on ADAS-cog delayed recall (β=-1.00, pFDR<0.001) and MMSE (β=-1.01, pFDR<0.05) compared to the MTL-sparing and Limbic-subtype over time, respectively.
Discussion: In a large single-site dataset, we confirm and extend findings that spatiotemporal subtypes of tau burden are differentiated by demographics and risk-factors. Cortical atrophy patterns are mostly consistent with the distribution of tau burden. The Lateral-Temporal-subtype showed higher tau burden and increased CSF markers of neurodegeneration, in line with the volume loss and cognitive decline.
Figures:
Authors & Affiliations:
Jacob Vogel1, Lyduine Collij2,3, Sophie Mastenbroek2,3, Alexa Pichet Binette3, Ruben Smith3,4, Sebastian Palmqvist3,4, Niklas Mattsson-Carlgren3,4, Olof Strandberg3,4, Rik Ossenkoppele3,5, Oskar Hansson3,4
1 Department of Clinical Science Malmö, SciLifeLab, Lund University, Lund Sweden
2 Amsterdam UMC, Department of Radiology and Nuclear Medicine, Amsterdam, the Netherlands
3 Clinical Memory Research Unit, Department of Clinical Sciences Malmö, Lund University, Lund, Sweden
4 Memory Clinic, Skåne University Hospital, Malmö, Sweden
5 Amsterdam UMC, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam, The Netherlands